Reactivity of Silyloxonium Ions with Palladium(0) Nucleophiles: Harnessing a Pd–Silyl Cation
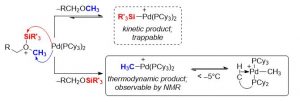
Ongoing research involving Pd(0) nucleophiles aims to study their reactivity towards electrophilic silyloxonium ions and explore how this reactivity to can be harnessed to utilize inert ethereal C–O bonds as substrates in transition metal catalyzed C–C bond forming reactions. Current work has discovered that silyloxonium Si–O bond cleavage is kinetically preferred and reversible, while silyloxonium C–O bond cleavage is thermodynamically preferred and irreversible. The putative LnPd–SiR3+ species expected to result from Si–O bond cleavage has been successfully trapped, while the LnPd–Me+ species resulting from Me–O bond cleavage has been spectroscopically observed at low temperatures. Current work aims to explore the reactivity of the highly reactive, yet easily generated L2Pd–SiR3+ cation towards dialkyl ethers as well as developing methods to utilized this complex to catalytically convert ethereal C–O bonds to C–H and C–C bonds.
- “Silylpalladium Cations Enable the Oxidative Addition of C(sp3)–O Bonds.” Wierschen, A. L.; Romano, N.; Lee, S. J.; Gagné, M. R. J. Am. Chem. Soc. 2019, 141, 16024–16032. Link
- “Silylpalladium Cations Enable the Cleavage of Nitrile C–CN Bonds,” Wierschen, A. L.; Lowe, J.; Romano, N.; Lee, S. J.; Gagné, M. R. Organometallics 2020, 39, 1258-1268. Link